Moderna, Inc.: The Latest Pullback Is No Purpose To Accumulate (NASDAQ:MRNA)
Funding Thesis
The much-awaited peer-reviewed information of mRNA-1273, the COVID-19 vaccine candidate of Moderna, Inc. (MRNA), has left many questions unanswered. Although the vaccine has been secure and produced neutralizing antibodies, the Section three trial will search for the vaccine’s medical efficacy, which relies on the comprehensiveness of the immune response it elicits. In comparison with a rival candidate utilizing the identical know-how, the cell-mediated immune response generated by mRNA-1273 appears to be like weaker. The late-stage trials scheduled to start out this week may reveal main hostile occasions missed within the a lot smaller Section-1 trial. In the meantime, the latest patent dispute has put into doubt the income potential of Moderna’s whole vaccine platform.
Nonetheless, the corporate stays well-funded to proceed with the late-stage research, and the rivals have signed up offers price billions of {dollars} to provide their experimental vaccines to governments as early as subsequent September. The low cost within the present buying and selling a number of has failed to completely seize the most recent uncertainties, and due to this fact, we keep away from additional accumulation of the inventory forward of the dangerous Section three research. Taking a leaf out of presidency’s COVID-19 playbook, it is at all times higher to diversify the bets by investing in a number of vaccine builders advancing a wide range of applied sciences.

Supply: Moderna – Vaccine Day Presentation April 2020
The Race to Eradicate the Pathogen
The raging COVID-19 pandemic has intensified the humanity’s resolve to discover a vaccine towards SARS-CoV-2, the novel coronavirus inflicting the illness. On the time of my final article on Moderna, solely eight vaccine candidates had reached the medical levels. Now 24 candidates are within the fray highlighting the competitiveness and monetary beneficial properties obtainable for front-runners. mRNA-1273, the vaccine candidate being developed by Moderna in collaboration with the NIAID (Nationwide Institute of Allergy and Infectious Ailments) of the NIH (Nationwide Institutes of Well being), was the primary to achieve human trials in March. A collection of catalysts in its improvement, together with the discharge of constructive top-line information of the Section 1 trial final Might, has greater than tripled Moderna’s share value this 12 months, whereas the NASDAQ Biotechnology Index (NBI) has gained solely ~13%. With extra candidates displaying promising leads to their early-stage research, Moderna’s share value had plummeted final week and appears much more weak as mRNA-1273 enters the late-stage trials this week amid an ongoing Section 2 trial.
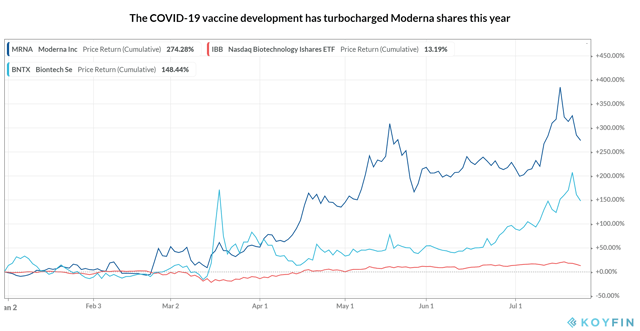
Supply: Koyfin
The LNP Supply System
Moderna’s vaccine know-how makes use of mRNA (ribonucleic acids) that instruct the human cells to provide the coronavirus Spike protein. As soon as recognized as a international agent by the human immune system, it primes the physique to mount a fast and efficient immune response to battle the virus when the an infection happens. Nonetheless, after the injection, the blood can break down RNA throughout the supply section and cell membranes can even act as a barrier to the compound’s mobile entry. To deal with the difficulty, Moderna makes use of the LNP (lipid nanoparticle) supply system. As soon as injected, the LNPs containing the mRNA first bind to proteins that promote the mobile uptake of the particles, which then launch the mRNA throughout the cell. A collection of catalysts in mRNA-1273 improvement, together with the discharge of constructive top-line information of the Section 1 trial final Might, has greater than tripled Moderna’s share value this 12 months, whereas the NASDAQ Biotechnology Index has gained solely ~13%. With extra candidates displaying promising leads to their early-stage research, Moderna’s share value had plummeted final week and appears much more weak as mRNA-1273 enters the late-stage trials this week amid an ongoing Section 2 trial.
The Rivals Are Equal to The Activity
In the meantime, the rival candidates have demonstrated security and constructive trial information of their early-stage research. This month, Pfizer Inc. (PFE) and BioNTech SE (BNTX) introduced favorable preliminary information from two separate Section half of trials of BNT162b1, one in every of 4 experimental COVID-19 vaccine candidates co-developed by the businesses, utilizing comparable know-how to Moderna’s. In the meantime, based on the info revealed by the medical journal, The Lancet, AZD1222, co-developed by AstraZeneca PLC (AZN) and Oxford College, has proven constructive leads to one other Section half of medical trial. Primarily based on a weakened frequent chilly virus (adenovirus) in chimpanzees, AZD1222 is a recombinant vaccine containing the genetics for SARS-CoV-2 Spike protein. Nonetheless, utilizing the identical know-how, Ad5-nCOV, an experimental vaccine developed by CanSino Biologics Inc. (OTCPK:CASBF) and Beijing Institute of Biotechnology, has generated constructive information in what’s reported as the primary mid-stage COVID-19 vaccine research in a peer-reviewed journal. Nonetheless, the traders shouldn’t learn an excessive amount of into small-scale early stage-data, which primarily focuses on evaluating the security and the immune response of the experimental vaccines. Solely the larger late-stage trials will verify the security and assess the total efficacy of a candidate. Whereas BNT162b1 is about to bear its Section 2b/three research this week, the Section three research for AZD1222 have already began in Brazil, South Africa, and the U.Okay. The disparity within the vaccine research makes their comparability extremely speculative, a process greatest left to specialists. Nonetheless, the early-stage information of mRNA-1273 and BNT162b1 have uncovered a number of noteworthy variations.
Insufficient mobile Immune Response?
Earlier than exploring them, let’s take a short take a look at the human immune system, the pure protection mechanism towards the international our bodies, and disease-causing brokers. It consists of two parts: the innate immunity, made up of non-specific obstacles reminiscent of pores and skin, and the adaptive immunity that springs into motion as soon as the physique will get uncovered to a selected pathogen (antigen). The adaptive immunity, which will also be elicited by a vaccine, has the reminiscence to reinforce the following immune response. It consists of two parts: mobile Immunity and humoral Immunity. The mobile immunity drives the cell-mediated response from the mixed work of T Helper Cells (CD4+ T cells), Cytotoxic T cells (CD8+ T Cells), and Regulatory T cells. CD4+ T cells primarily activate different immune cells to battle the international agent. CD8+ T Cells kill the virus-infected cells and eliminates the pathogen, whereas the Regulatory T cells management the immune response from performing excessively. In humoral immunity, B Cells interacting with T Helper cells mount an antibody-mediated response.
Turning to vaccine candidates, all three have carried out properly on the humoral immunity entrance, producing SARS-CoV-2 neutralizing antibodies. Nonetheless, within the preliminary U.S. trial, BNT162b1 has generated the very best stage of neutralizing antibodies with a dose of solely 10 – 30 micrograms, properly beneath the 100 micrograms of mRNA1273, the optimum dosage chosen by Moderna for the Section three trial. Based on the COVID-19 vaccine approval standards set by the FDA lately, the presence of antibodies alone won’t assure the FDA approval, because the antibody stage required for defense towards COVID-19 stays unknown but. For approval, any profitable candidate must be secure and 50% more practical than the placebo. Nonetheless, as per the preliminary information, Moderna’s mRNA1273 appears to be like to have generated what appears to be an insubstantial mobile immune response eliciting solely a small elevation of CD8+ T Cells. Whereas the corporate attributed the lighter response to the shorter time interval of the research, BNT162b1 has managed to generate a strong CD4+ and CD8+ response inside 43 days.
A Authorized Dispute Provides Extra Confusion
Except for the Section 1 issues, a authorized setback has clouded the commercialization prospects of Moderna’s whole vaccine platform. The USPTO (U.S. Patent and Trademark Workplace) has turned down the corporate’s bid to invalidate a U.S. patent owned by Arbutus Biopharma Company (ABUS). Referred to as the ‘069 patent, it pertains to Moderna’s LNP know-how, the premise of its vaccine platform, designated as ‘proprietary’ by the corporate in its newest 10-Q submitting. Though the Moderna pressured the ruling wouldn’t have any influence on mRNA-1273 improvement, the traders weren’t satisfied, dragging the shares greater than ~9% and sending Arbutus ~119% larger on the day the information broke out. The issues are comprehensible because the ruling can put a dampener on Moderna’s progress story. If mRNA-1273 will get the regulatory nod, the royalty funds to Arbutus can price the corporate a couple of proportion factors of the vaccine gross sales.
Nonetheless, the corporate seems to be dedicated and well-funded to proceed with the large-scale Section three trial and manufacture 500 million – 1 billion doses of the vaccine from subsequent 12 months. Following an fairness supply price $1.three billion final Might, to scale up the manufacture of mRNA-1273, this week, the corporate introduced an settlement with BARDA (Biomedical Superior Analysis and Growth Authority) for an extra $472 million. Taking the federal government establishment’s whole grants for Moderna to $955 million, the most recent allocation meets the scarcity in funding for a a lot bigger Section three trial as required by the FDA. In the meantime, the rivals have cast provider agreements price billions of {dollars} with governments. As Pfizer/BioNTech goals to provide 500 million doses of its vaccine candidate by the year-end earlier than elevating the capability to 1.three billion doses subsequent 12 months, each the U.Okay. and the U.S. have lined as much as order 30 million and 100 million doses, respectively. Hailed because the ‘most superior’ vaccine candidate by the WHO’s chief scientific officer, AstraZeneca’s AZD1255 is focusing on to ship billions of doses by 2021, together with 300 million for the U.S. authorities. A contract for 100 million doses for the U.Okay. contains an settlement to satisfy 30 million doses as early as subsequent September.
Many Uncertainties Prompts Diversification
Though the constructive early-stage trials won’t assure profitable late-stage outcomes, the traders appear to have already picked the winners pricing them properly forward of the income potential. Because the contracts with the U.S. authorities point out, it’s too early to take a position on their pricing technique, not to mention estimate the income. Whereas the newest Pfizer/ BioNTech deal implies $19.50 per dose for BNT162, an settlement with Novavax, Inc. (NVAX) for 100 million doses of its vaccine candidate, at present in Section 1 trials, suggests a price ticket of $16.00 per dose. In contrast to AstraZeneca, whose provide of 300 million doses signifies a value of $4.00 per dose for AZD1255, each Pfizer and Novavax intend to make earnings from their vaccine applications. With out going by means of the detailed agreements, the pricing estimates primarily based on the face worth of the above contracts will solely be guesswork. Nonetheless, the uncertainty over pricing stays a problem although the consensus estimates counsel ~$1.6 billion in income for Moderna in 2020, greater than a 27-fold enhance from the extent in 2019.
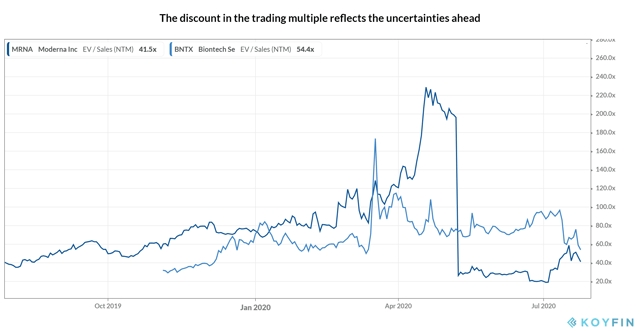
Supply: Koyfin
Whereas Moderna’s NTM EV/Gross sales a number of stands at ~41.5x with a reduction of ~43.5% to the previous 12 months common, it at present trades at ~17.4x when it comes to 2021 gross sales indicating ~41.0% low cost to BioNTech’s EV/Gross sales a number of of ~29.5x for the 12 months. The most recent patent dispute and the obvious incomplete immune response of mRNA-1273 seem to have solely partially mirrored within the low cost, discouraging a Bullish view on the inventory. As an alternative, with many candidates within the fray, it’s at all times higher to diversify the portfolio, as accomplished by the governments, by putting the bets on a number of builders following completely different applied sciences hoping at the very least one will succeed.
Conclusion
The peer-reviewed information of the Section 1 trial of Moderna’s COVID-19 vaccine candidate has generated recent issues over its immune response. Whereas a rival candidate utilizing the identical know-how managed to elicit a stronger and extra complete response, Moderna’s mRNA-1273 has solely generated a modest impact. Though the drug was confirmed to be secure to move into the late-stage trials beginning this week at a better dose in comparison with the rival, the latest patent dispute may hamper its income potential. Because the low cost within the buying and selling a number of partially mirrors the present uncertainties, we might keep away from additional accumulation of Moderna and as a substitute look to diversify the present COVID-19 vaccine portfolio.
When you loved this text and want to obtain updates on my newest analysis, click on “Observe” subsequent to my identify on the high.
Disclosure: I/we now have no positions in any shares talked about, and no plans to provoke any positions throughout the subsequent 72 hours. I wrote this text myself, and it expresses my very own opinions. I’m not receiving compensation for it (apart from from In search of Alpha). I’ve no enterprise relationship with any firm whose inventory is talked about on this article.
The post Moderna, Inc.: The Latest Pullback Is No Purpose To Accumulate (NASDAQ:MRNA) appeared first on Chop News.
from Chop News https://ift.tt/33dqcj9
Comments
Post a Comment